Copy-number Variation (Cnv) Can Be Produced by: (Select All That Apply.)
Introduction
One of the important challenges in post-genomic biology is relating observed phenotypic variation to the underlying genotypic variation. Genome-wide association studies (GWAS) have made thousands of connections between single-nucleotide polymorphisms (SNPs) and phenotypes, implicating regions of the genome that may play a causal role in a diverseness of complex traits. Despite their success in identifying associated variants, association studies business relationship for merely a pocket-sized pct of the total heritability (Maher, 2008). Hence, determining other types of variation that may make a substantial contribution to variation in circuitous traits is a meaningful goal.
Copy number variations (CNVs) are gains and losses of big regions of genomic sequence betwixt individuals of a species, ranging from kilobases to megabases in length (Feuk et al., 2006). It is hypothesized that CNVs represent a pregnant source of genetic variation, as they accept been shown to cover approximately 7% of the mouse genome (Locke et al., 2015), 12% of the human genome (Redon et al., 2006), and seven% of the cattle genome (Keel et al., 2016a). Meaning overlap between protein-coding genes and CNV has been reported in a number of species, including man (Bailey et al., 2009), mouse (Locke et al., 2015), cattle (Keel et al., 2016b), and pig (Paudel et al., 2013). Conrad et al. (2010) constitute that twoscore% of validated CNV overlapped with at least 1 factor. In addition, CNVs appear to influence gene expression levels (Stranger et al., 2007; Henrichsen et al., 2009).
In humans and rodents, CNVs have been well studied and linked to various phenotypic traits and diseases (Cook and Scherer, 2008; Almal and Padh, 2012; Girirajan et al., 2013). Initial CNV studies accept been performed in a number of domesticated animals: canis familiaris (Nicholas et al., 2011; Alvarez and Akey, 2012; Berglund et al., 2012), cattle (Fadista et al., 2010; Liu et al., 2010; Hou et al., 2011; Stothard et al., 2011; Zhan et al., 2011; Bickhart et al., 2012; Hou et al., 2012a; Hou et al., 2012b; Jiang et al., 2012; Choi et al., 2013; Wu et al., 2015; Keel et al., 2016a), sheep (Fontanesi et al., 2011; Liu et al., 2013), craven (Crooijmans et al., 2013; Yi et al., 2014), and caprine animal (Fontanesi et al., 2010).
Swine CNVs have been reported using a diversity of array-based platforms, including comparative genomic hybridization arrays (Fadista et al., 2008; Li et al., 2012; Wang et al., 2014; Wang J. et al., 2015), the Illumina PorcineSNP60 BeadChip (Ramayo-Caldas et al., 2010; Chen et al., 2012; Wang et al., 2012; Wang L. et al., 2013; Schiavo et al., 2014; Wiedmann et al., 2015; Xie et al., 2016; Zhou et al., 2016; Hay et al., 2017), and the Illumina Infinium II Multisample SNP assay (Wang J. et al., 2013; Long et al., 2016). These approaches are known to suffer some drawbacks, including express coverage of the genome due to low probe density, low resolution, and hybridization noise (Zhao et al., 2013). Ongoing developments and cost decreases in next-generation sequencing (NGS) technology have led to an increased popularity of sequence-based CNV detection. To appointment, a express number of studies have utilized NGS data to identify CNV in the porcine genome.
The number and size ranges of CNV detected in previous swine studies utilizing NGS vary dramatically. These discrepancies may be artifact of differences in many aspects of the study, including sequence coverage, sample size, breed, and CNV detection algorithm. In swine, as well as many other species, relatively little is known most the properties of CNV, including their frequency in the genome, sizes, locations, and chromosomal properties. Of all the topics related to CNV, knowledge of their functional impact is the most limited. Despite the broad range of number and size of CNV reported between previous swine NGS studies, the results from functional enrichment analysis of CNV are quite consistent. Gene ontology (Go) terms related to sensory perception (Paudel et al., 2013; Jiang et al., 2014; Paudel et al., 2015), response to stimuli (Paudel et al., 2013; Jiang et al., 2014), immunity (Jiang et al., 2014; Paudel et al., 2015), and olfactory receptor (OR) activity (Paudel et al., 2015; Revilla et al., 2017) were the about significant in these studies. The same GO terms have been identified in CNV studies in humans and cattle. ORs, which are 1000-protein-coupled receptors involved in signal transduction, play a function in all the Become terms listed above. The results from previous studies suggest that CNVs may play a function in olfactory ability and sensitivity, which may be related to economically relevant traits in swine including feeding beliefs (Connor et al., 2018) and reproduction (Baum and Blood-red, 2015).
The CNVs reported in the aforementioned studies stand for several diverse hog breeds and wild boars from different regions of the earth. Very few animals in these studies (merely 37 of 353) represent commercial swine germplasm, which, through domestication, has been shaped by selection for docility and lean meat product. Additionally, previous CNV studies in swine have been conducted using the Sscrofa ix.two and x.2 genome builds. The purpose of this study is to identify and narrate CNV regions detected from whole-genome sequence of 240 members of an experimental swine herd at the U.Southward. Meat Animal Research Center (USMARC), a resource representative of commercial swine germplasm, utilizing the newly released, high-quality Sscrofa xi.1 genome associates.
Materials and Methods
The Deoxyribonucleic acid samples sequenced for this report were extracted from semen, claret, and tail tissue archived under standard operating procedures for the U.S. Meat Animal Research Center tissue repository. The inquiry did non involve experimentation on animals requiring IACUC approving.
Sequencing and Data Acquisition
CNVs were detected from whole-genome sequence of 240 members of an experimental swine herd. This composite population, adult at USMARC, began in 2001 by mating mixed Landrace-Yorkshire sows with 24 purebred founding boars—12 Landrace and 12 Duroc. To produce the second generation, Landrace-sired animals were mated to Duroc-sired animals. Subsequent generations were produced by selecting 1 male person and ten females produced by each founding boar then randomly mating them, avoiding total-sib and half-sib pairings (Lindholm-Perry et al., 2009). Industry sires were then introduced in generation 10 and used in subsequent generations. This study utilizes whole-genome sequence from all 24 founding boars, 48 of the founding sows, and 109 animals from generations 4 through nine, 29 animals from generation 15, and thirty purebred industry boars (15 Landrace and fifteen Yorkshire) used as sires in generations 10 through 15.
Deoxyribonucleic acid extraction and library preparation have been previously described in Keel et al. (2017) and Keel et al. (2018) for the 72 founding animals and the remaining 168 animals, respectively. Libraries were paired-end sequenced (150 bp read length) on an Illumina NextSeq500 (Illumina, San Diego, CA, USA) at USMARC. Bases of the paired-cease reads for all sequenced genomes were identified with the Illumina BaseCaller, and FASTQ files were produced for downstream analysis of the sequence data. Sequence data are available for download from the National Middle for Biotechnology Information (NCBI) Sequence Read Archive (SRA) BioProjects PRJNA343658, PRJNA414091, and PRJNA482384.
Sequence Data Processing
The Trimmomatic software (Version 0.35; Bolger et al., 2014) was used to trim Illumina adaptor sequences and low-quality bases from the reads. The quality cutoff was a PHRED33 score of >15. Reads containing any portion with an average PHRED33 score <xv spanning at least 4 bp were removed. The remaining reads were mapped to the Sscrofa 11.1 genome associates using Burrows-Wheeler Alignment (BWA, Version 0.7.12; Li and Durbin, 2009) with the default parameters.
CNV Detection and Defining CNVRs
A combination of the CNVnator (Version 0.3.2; Abyzov et al., 2011) and LUMPY (Version 0.4.13; Layer et al., 2014) software was used to identify putative CNV in the genome sequence of the 240 pigs. LUMPY is a probabilistic CNV discovery framework that integrates multiple detection signals, including split reads and paired-end mapping, while the CNVnator is a read depth method that uses a mean-shift-based approach to phone call CNV based on the depth of sequencing.
CNVs were first called for each sample using the CNVnator. The program was run using a window size (bin size) of i kb, and all other parameters were set to the default. Next, CNVs were detected using LUMPY with default parameters. CNV breakpoints from the CNVnator output were passed as input into LUMPY using the –bedpe option.
In an attempt to reduce the number of false positives, CNVs were as well called using the cn.MOPS algorithm (Version 1.24.0; Klambauer et al., 2012). cn.MOPS is a multiple sample read depth method that applies a Bayesian approach to decompose read variations across multiple samples into integer copy numbers and noise by its mixture components and Poisson distributions, respectively. cn.MOPS avoids read count biases along the chromosomes past modeling the depth of coverage across all samples at each genomic position. The cn.MOPS programme was run using a window length of 1 kb, mean normalization mode, and the default values for all other parameters. Autosomal and sex activity chromosomes were processed differently due to differences in expected ploidy of the genome. Autosomal CNVs, which are expected to be diploid, were identified using all 240 samples. CNVs on the sex chromosomes were identified by processing the 167 males and 73 females separately, as SSCX is expected to exist diploid in female samples and SSCX and SSCY are expected to be haploid in the male samples.
CNVs identified by LUMPY that were at least 10% overlapped by a CNV identified past cn.MOPS, pregnant that the ratio of the number of bp overlapped betwixt the LUMPY CNV and at to the lowest degree i cn.MOPs CNV to the length of the LUMPY CNV was greater than 0.10, were retained for downstream assay. Next, CNVs were used to construct a fix of copy number variable regions (CNVRs). A CNVR was synthetic by merging CNVs beyond samples that exhibited at least 50% pairwise reciprocal overlap in their genomic coordinates. For example, suppose nosotros take 2 CNVs, CNV1 beginning at position a and ending at position b and CNV2 running from c to d with a < c < b < d. If the reciprocal overlap between the two CNVs is at least l%, then they are merged into a CNVR that runs from a to d on the genome.
Validation of CNVR Using Data From Sequenced Parent–Offspring Trios
For the transmission charge per unit (paternal and maternal), in each parent–child pair, CNVRs in the parent also chosen in the child were counted so divided by the total number of CNVR calls in the parent. For the inheritance rate, CNVR calls in the child too present in at least one parent were counted and and then divided by the total number of CNVRs in the child.
Gene Content and GO
Genes from the NCBI Sus scrofa annotation (Release 106) overlapping by at least ane bp with CNVRs were identified. Functions of protein-coding CNV-overlapped genes were determined using the PANTHER nomenclature system (Version fourteen.0, Mi et al., 2013).
Enrichment assay of factor function was performed using PANTHER'southward implementation of the binomial test of overrepresentation. Significance of Become terms was assessed using the default Sus scrofa GO annotation as the reference set for the enrichment analysis, and data were considered statistically pregnant at a Benjamini-Hochberg-corrected P value < 0.05.
Enrichment of Quantitative Trait Loci
Enrichment analysis of quantitative trait loci (QTL) overlapped with CNVR was performed using Fisher'due south exact test. Data were considered statistically significant at a Benjamini-Hochberg-corrected P value < 0.05.
Results and Discussion
Sequencing and Read Mapping
Genomic DNA from 240 pigs, from a composite population at USMARC, was sequenced on Illumina HiSeq and NextSeq platforms, generating approximately 72 billion paired-end reads (Tabular array S1). Sequence reads covered each squealer's genome at a mean of 13.62-fold (×) coverage. Individual coverage per animal ranged from 0.97× to 31.13×; 24 animals were covered at less than 3×, and 44 were covered at more 20×.
When generating our sequence data, we targeted a minimum of iii× coverage for each of the founding sows and 10× coverage for the remaining 168 animals. However, there was considerable variation around the 3.66× and 18.41× mean coverage for the founding sows and other animals, respectively. Some of this variation can be attributed to technical aspects of NGS technology, such equally the stochasticity of sequencing, Dna quality, and library preparation. The combined sequence from all 240 animals covered 99.99% of the reference genome.
CNVR Discovery and Statistics
CNVs in the genome of the 240 pigs were identified by taking the overlap of two methods: (1) a combination of CNVnator and LUMPY and (2) cn.MOPS. Virtually of the previous NGS CNV studies in swine have utilized read depth approaches to identify variants (Paudel et al., 2013; Jiang et al., 2014; Paudel et al., 2015; Wang H. et al., 2015; Wang et al., 2016; Revilla et al., 2017). Although read depth can be a powerful tool to place CNV, oft the boundaries are not well determined because of the sliding window approach. The exact boundaries of CNV events tin exist important for determining their functional effect (e.thou., affecting coding sequence). Other CNV detection strategies, such equally paired-finish mapping or split up reads, tin can be used to fine map CNV and determine more precise boundaries of the variants. The CNVnator–LUMPY combination approach used in this piece of work calls CNV in private samples utilizing paired-terminate mapping, split reads, and read depth. Although this method should give more accurate CNV breakpoints than read depth bespeak alone, single sample CNV callers are known to suffer from decreased detection power and loftier false-positive rates. A total of 2,079,579 were identified using CNVnator–LUMPY. Utilization of data from multiple samples has been shown to better CNV detection (Klambauer et al., 2012; Duan et al., 2014). Therefore, equally a further fault correction step, CNVs were also detected using a multiple sample read depth caller, cn.MOPS (695,741 CNV identified). A total of 39,315 CNVs, overlapping between the two methods, were retained for downstream analysis (Tabular array S2). CNVs were merged beyond each genome then across samples into CNVRs, and CNVRs less than 200 bp in length were filtered out. This resulted in a final set consisting of iii,538 CNVRs (Tabular array S3), including i,820 novel CNVRs that were not reported in previous studies.
Note that approximately 19% (45 of 240) of the animals in this study had low to moderate sequence depth (<5× coverage). The highest sensitivity and resolution in CNV detection are attained through high coverage sequencing (>10×; Alkan et al., 2011). However, until sequencing costs drop dramatically, it is not viable, in nearly cases, to generate high coverage genomic sequence on large numbers of animals. We consider low-coverage sequencing data hither, considering methods for analyzing SNP and CNV in depression-coverage information volition proceed to exist relevant in the future in terms of a study'due south discovery power, where a stock-still number of reads should rather be used for sequencing more samples with lower coverage than for sequencing fewer samples with higher coverage (Le and Durbin, 2011). Due to the cost-effectiveness of sequencing at lower coverage, recent studies have explored strategies for using low-coverage sequence to detect mutual CNV that could explain a pregnant amount of phenotypic variation (Keel et al., 2016a; Zhou et al., 2018). Both CNVnator and cn.MOPS have been shown to have moderate to high accurateness in detecting CNV from low-coverage sequence in diploid genomes (Keel et al., 2016a; Malekpour et al., 2018), particularly in information sets consisting of samples with mixed levels of coverage. Therefore, the utilize of these methods, coupled with LUMPY, should provide reasonably accurate results for CNV calling in our 240 animals.
Sizes of the CNVRs ranged from 0.203 to 398.9 kb, with an average of 6.eight kb and a median of 2.ix kb. The CNVR occupied a total of 22.ix unique Mb or 0.94% of the Sus scrofa genome. The CNVR coverage of the genome is lower than the results of previous reports in swine (4.0%; Jiang et al., 2014) and other species, including mouse (6.87%; Locke et al., 2015), human (12%; Redon et al., 2006), and cattle (vi.7%; Keel et al., 2016a), which may exist due to our stringent criteria (e.g., requiring detection with two unlike approaches). Among the CNVRs, 144 showed copy number gain (duplication), 3372 showed re-create number loss (deletion), and 22 showed a mix of copy number loss and proceeds from different individuals. Clearly, there was a big discrepancy in the numbers of duplication and deletion CNVR. Overall, read-depth methods are more sensitive to deletion CNV calls than duplication calls, especially in mid- to depression-coverage sequence data, as it is easier to identify a "missing" segment of the genome than an amplified one with limited sequence reads. In fact, iii.1% (105 of 3372) of deletion calls were identified in merely animals with <10× coverage. As low-coverage WGS continues to go more widely utilized, it volition exist necessary to focus on adapting CNV calling tools to this type of information.
Distribution of CNVR
The distribution of CNVRs along each of the chromosomes is shown in Figure 1. Variants were not uniformly distributed on the chromosomes. The number of CNVRs was strongly correlated with the size of the chromosome (Pearson correlation coefficient r = 0.77). SSC1 and SSC13 exhibited the largest numbers of CNVRs (1231 and 231, respectively), while SSCY, SSC18, and SSC12 had the smallest numbers (2, 49, and 52 CNVRs, respectively). On average, 0.79% of each chromosome was covered by CNVRs (Table 1).
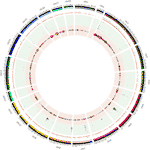
Figure 1 Positions of CNVRs identified from the 240 sequenced swine genomes in Circos format (Krzywinski et al., 2009). The outer ideogram runs clockwise from chromosome 1 to chromosome Y with labels in Mb of physical distance. The copy number data are represented in the inner tracks. The two innermost tracks show scatter plots of the CNVR, where the ruby track shows copy number loss and the green runway shows copy number gain. Concentric circles within these tracks indicate y-centrality values in the besprinkle plot. The 10 concentric circles in the red rail mark values 0 ≤ y < 2, with 0 existence the innermost rail, while the 11 concentric circles in the green track marker values 2 ≤ y ≤ 8. The size of the dot in the scatter plot is proportional to the number of samples containing the CNVR. The other track shows a heat map that indicates the parts of the genome that contain re-create number gain and loss. This plot just collapses the scatter plot values onto a single radial position.

Tabular array ane CNVR distribution across the genome.
The number of CNVRs per beast ranged from 0 to 348, with a mean of 157.viii (Table S4). CNVs spanned up to 0.13% of the genome of each animal, with a mean and median of 0.057% and 0.062%, respectively. This variation across individuals can be partially explained by differences in genomic sequencing coverage. Smaller numbers of CNVRs were identified in samples with low sequencing depth, and the number of identified CNVRs tended to increase every bit genomic coverage increased (Pearson correlation coefficient r = 0.84).
The number of individuals exhibiting each CNVR ranged from one to 175. Many CNVRs (∼2649) were present in a small percentage (< 5%) of the animals. Iii CNVRs (CNVR 2103, 1676, and 2104 in Tabular array S3) were nowadays in more 60% of the population. The distribution of deletion, duplication, and mixed CNVRs across breeds is shown in Figure ii. The purebred Landrace and Yorkshire boars and the composite animals had more CNVR of all three types than the purebred Duroc boars. This is likely because the Sscrofa eleven.1 reference genome assembly was obtained from a Duroc animal.
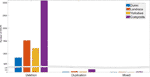
Effigy 2 Distribution of CNVR types across breeds.
A total of 1620 CNVRs were found to exist breed-specific in origin (Table S5). Most (64%) breed-specific CNVRs were present in only 1 animal. Breed-specific CNVRs that were present in the largest numbers of animals were found in the composite brood. This is probable due to the larger number of composite animals in the information set. Increased numbers of animals in the three pure breeds volition be necessary to conduct a complete breed-of-origin analysis. This will be a focus of future work.
CNVR Concordance in Parent–Offspring Trios
Pedigree data from 12 sequenced parent–offspring trios were used as a substitute for molecular validation, which nosotros have chosen to forego since this work was intended to be a discovery. In a follow-up study, we are planning to look for CNVRs that may associate with phenotypes in our population and validation using PCR methods volition be performed for those CNVRs. If a CNV is transmitted from parent to offspring, then information technology tin can be considered validated. Although this type of validation is not 100% authentic, it is satisfactory to allow u.s.a. to guess error rates. In an ideal data fix, paternal and maternal transmission rates would exist 50%, and inheritance rates would be 100%. Deviations from this ideal could be explained by multiple factors. Both false-positive and fake-negative CNV calls will cause a decrease in transmission and inheritance rates. Another possible gene is de novo mutations in offspring, which volition not affect transmission rates, but volition affect inheritance rates. Additionally, there is the possibility of somatic mutation in one or more of the parents, essentially a de novo mutation in parents as they age. Somatic mutations would bear on transmission rates only not inheritance rates. All of these factors could potentially affect the data simultaneously. Therefore, they cannot exist individually estimated. Withal, bold that de novo and somatic mutations are rare compared to CNVR calling errors, nosotros can use manual and inheritance rates to estimate error rates.
Table 2 shows the paternal and maternal transmission rates and the inheritance rate for each of the 12 sets of trios. The boilerplate transmission rates were 37.7% and 41.4% for maternal and paternal, respectively. These rates are much closer to the ideal 50% transmission rate than what was reported in a similar study in humans (27% for maternal and 28% for paternal; Zheng et al., 2012). The boilerplate inheritance rate was 52%, which falls between inheritance rates reported in previous studies, 42% in Zheng et al. (2012) and 74.8% in Wang et al. (2007). Therefore, nether the assumption that the de novo and somatic mutations are rare, nosotros approximate the error rate in CNVR calls to exist 48% (100% minus the inheritance rate). This error rate is comparable to previously published results for several unlike CNV-calling algorithms for whole-genome sequence data (26%–77%; Legault et al., 2015). This consistency suggests that high fault rates may be due to algorithmic issues rather than the input data. Clearly, further evolution of bioinformatics protocols and tools for producing high-confidence, consistent CNV calls is necessary to improve the quality of CNV discovery studies.
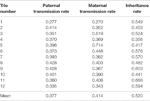
Tabular array 2 Transmission and inheritance rates in parent–offspring trios.
Comparison of CNVRs with Previous Studies
Comparing of our results with CNVRs identified in several previous swine studies showed varying levels of overlapping CNVRs between studies (Tabular array 3). Here, nosotros used a much less stringent definition of overlap than that used in identifying overlapping CNV, where two CNVRs were considered overlapped as long equally they shared at least ane base.

Table 3 Comparison of CNVRs identified in this study to results from other studies (based on the Sscrofa 11.one genome assembly).
More often than not speaking, percentages of overlap in CNV events identified between this work and previous studies were low (average of 4.33% overlap). This issue is very similar to what has been observed in cattle CNV studies, where typically <twoscore% overlap exists between studies (Keel et al. 2016b). These discrepancies are likely driven by many technical aspects of the experiments, including vastly dissimilar sample sizes, differences in breeds and the number of breeds represented, detection platform (assortment-based vs. NGS), and CNV detection algorithms. Many of the CNV discovery studies in swine take involved pure and half Chinese breeds. Therefore, it is probable that many of the CNVRs identified in those studies practice non segregate in our population. Our population represents commercial swine germplasm and, considering of domestication and selection for lean meat production and reproductive efficiency, has diverged from germplasm studied in other experiments.
It should be noted that two of the three studies with highest overlap percentages, Wang et al. (2013) and Wiedmann et al. (2015), were those that had high representations of Yorkshire, Landrace, and Duroc animals. In fact, the study of Wiedmann et al. (2015) was performed on animals from the same population used in this study. The discrepancy in CNV identified in their study and ours is probable due to differences in platform (SNP beadchip vs. whole-genome sequence), detection algorithm, and genome build (Sscrofa 10.2 vs. Sscrofa 11.1).
Function of CNV-Overlapped Genes
A total of 1401 genes from the NCBI annotation of the Sscrofa eleven.ane genome were identified to exist overlapping with our detected CNVRs (Table S3), including 911 protein-coding genes, 58 pseudogenes, 273 non-coding RNA, and 160 miscellaneous RNA. CNV-overlapped genes were overlapped with 2314 CNVRs. Using PANTHER's functional note tool to inspect GO slim terms mapping to protein-coding CNV-overlapped genes, we identified that many of these genes were involved in binding (34.7%), catalytic activity (35.7%), metabolic process (23.ane%), biological regulation (20.3%), cellular process (xi.four%), localization (nine.three%), and molecular transducer activeness (9.2%).
Enrichment analysis was performed, using the Sus scrofa Get database to identify GO terms that were significantly enriched in our factor gear up. Become enrichment assay showed that biological procedure terms related to regulation of ion transport, cell adhesion, signaling, nervous organization development, neurogenesis, and locomotion, as well as molecular function terms related to glutamate receptor activity, protein binding, enzyme bounden, ATP binding, and neurotransmitter receptor activeness, were significantly overrepresented in the genes overlapped by CNVR (Benjamini-Hochberg-corrected P value <0.05; Table S6).
Approximately three.6% of the CNV-overlapped genes belonged to the OR gene family, one of the largest gene families in the porcine genome (Groenen et al., 2012; Nguyen et al., 2012). ORs are Chiliad-protein-coupled receptors involved in point transduction and have been plant to be copy number variable in many mammalian species, including man (Young et al., 2008), rat (Guryev et al., 2008), mouse (Pezer et al., 2015), swine (Chen et al., 2012; Wang et al., 2012; Paudel et al., 2013; Wang J. et al., 2013; Paudel et al., 2015), and cattle (Liu et al., 2010; Keel et al., 2016b; Xu et al., 2016). Young et al. (2008) showed that OR genes displayed varying copy numbers amidst 50 people, and that this variation may play a role in olfactory ability and sensitivity. It is also thought that ORs may play a chemosensory office every bit they are expressed on sperm and thought to direct them to the egg via chemotaxis (Spehr et al., 2006). Paudel et al. (2015) identified that OR genes were overrepresented amidst CNVRs across several members of the Sus genus. These genes may accept been important components of swine evolution, as scent would have been critical for foraging for food, avoiding predators, and finding a mate.
Overlap and Enrichment of Known QTL in CNVRs
To reveal the potential relationships between CNVRs and QTL, we analyzed the overlap betwixt our CNVRs and known swine QTL and performed QTL enrichment analyses. Swine QTL from the Sscrofa eleven.1 genome build were downloaded from the Brute QTL database (Release 34; http://www.animalgenome.org/cgi-bin/QTLdb/SS/index), which includes 26,076 known QTL for 647 different traits. QTL overlapping with CNVRs were identified (Tabular array S7A), traits were ranked according to the number of QTL/CNVR overlaps (Table S7B), and QTL enrichment analysis was performed (Table S7B). The 10 highest ranked traits included baste loss (519 overlaps), average daily gain (235 overlaps), average backfat thickness (195 overlaps), loin muscle area (179 overlaps), backfat at last rib (153 overlaps), teat number (127 overlaps), carcass length (95 overlaps), ham weight (81 overlaps), backfat at tenth rib (75 overlaps), and lean meat percentage (73 overlaps).
QTL enrichment assay, using QTL from the Animal QTL database overlapping with CNVR (due north = 525 traits), identified that QTL for 132 traits were significantly enriched. The nigh significantly enriched QTL was baste loss (P = iv.09E−99). Several meat quality traits, including boilerplate back fatty thickness, loin muscle area, ham weight, carcass weight, and dressing pct, were also found to be amid the about significantly enriched.
Approximately 840 QTL have been previously reported from GWAS utilizing animals from the same experimental herd used in this study (Table 4). QTL/CNVR overlaps were identified (Table S8A), traits were ranked according to the number of overlaps (Table S8B), and QTL enrichment analysis was performed (Table S8B). The highest ranked traits included vertebra number (28 overlaps), as well as several reproductive traits including age of puberty (41 overlaps), ovulation charge per unit (18 overlaps), % stillborn ignoring the final piglet (18 overlaps), and last birth interval (17 overlaps). It should be noted that, in this piece of work, CNVRs were not tested for statistical clan with QTL, but rather the overlapping genomic positions of the latter were used as i indicator of the potential function of the CNVRs.

Table 4 QTL identified in USMARC swine population from previously published GWAS.
Of the twenty GWAS traits that had QTL overlapping with CNVR, 7 of them were found to be significantly enriched. These included vertebra number (P = four.35E−07), percent stillborn ignoring the last piglet (P = 4.64E−07), last birth interval (P = 2.79E−06), number stillborn in the last nativity position (P = 1.58E−05), litter boilerplate nascence interval minus the last birth (P = eight.13E−05), kyphosis (P = 1.49E−05), and number stillborn ignoring the terminal piglet (P = ane.49E−04).
These results are similar to those from a study conducted by Revay et al. (2015), where age of puberty and teat number were establish to exist the most abundant reproductive QTL overlapped by swine CNVRs. This coupled with the overrepresentation of GO terms such equally cell motility, nervous system development, and organ development in CNVR-overlapped genes suggests that CNVR may play a function in shaping various reproductive traits in swine.
Conclusion
CNV continues to proceeds considerable involvement equally a source of genetic variation that may play a role in phenotypic multifariousness. Swine CNV research has made meaning progress in the last five years. Genome-wide surveys of CNV have been conducted using a diversity of platforms and algorithms. Studies that utilize NGS data take been express in swine. Moreover, much of the NGS-based studies accept focused on diverse pig breeds and wild boars from different regions of the world rather than commercial breeds. To capture CNV present in commercial swine germplasm, we utilized whole-genome sequence from 240 animals. Our written report is one of the largest sequence-based swine CNV studies to date.
We identified 1401 genes overlapping with CNVRs. Get enrichment assay showed that our fix of CNV-overlapped genes was enriched with genes involved in organism development, and QTL assay showed that CNVRs overlapped with many QTL for reproductive traits. These results are consistent with findings in other swine CNV studies, which suggests that CNV may play a role in shaping reproductive traits. Agreement the exact role that CNV plays in reshaping gene structure, modulating factor expression, and ultimately contributing to phenotypic variation are open questions. The focus of our future piece of work volition be to develop strategies for CNV imputation, identify CNVs that associate with phenotypes in our population, and validate those CNVs using methods such equally digital droplet PCR, with the long-term goal of discovering the extent to which CNVs bear upon economic traits of interest and developing strategies for incorporating them into genomic selection systems.
Data Availability
The raw data supporting the conclusions of this manuscript will be made bachelor by the authors, without undue reservation, to whatever qualified researcher.
Disclaimer
Mention of merchandise names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the footing of race, color, national origin, age, disability, and where applicable, sex, marital condition, familial status, parental status, faith, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual's income is derived from whatsoever public aid program. (Not all prohibited bases utilise to all programs.) Persons with disabilities who crave alternative ways for advice of program information (Braille, large impress, audiotape, etc.) should contact USDA'southward TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Managing director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, D.C. 20250-9410, or telephone call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer.
Author Contributions
BK conceived the report, and BK, DN, and GR participated in its blueprint and coordination. DN, GR, WO, and AL-P were involved in the acquisition of data, and BK performed all information assay. BK drafted the manuscript, and DN, GR, WO, and AL-P contributed to the writing and editing. All authors read and approved the final manuscript.
Disharmonize of Involvement Statement
The authors declare that the enquiry was conducted in the absence of any commercial or fiscal relationships that could exist construed as a potential disharmonize of interest.
Acknowledgments
The authors would like to give thanks Kris Simmerman for sample collection, Deoxyribonucleic acid extraction, and library preparation; Sue Hauver for library training; the USMARC Core Laboratory and the Iowa State Dna Core facility for performing the sequencing; and Rebecca Anderson for her assistance with the project during her summer internship.
Supplementary Fabric
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.00737/full#supplementary-material
Table S1 | Sequencing statistics.
Table S2 | CNV overlapping between Lumpy and cn.MOPS analyses.
Tabular array S3 | CNVR and their overlapping genes.
Tabular array S4 | Distribution of CNVR in individual animals.
Table S5 | Breed specific CNVR.
Table S6 | Results from GO enrichment analysis.
Table S7 | Results from QTL enrichment assay using QTL from the AnimalQTL Database.
Table S8 | Results from QTL enrichment analysis using previously identified QTL in the USMARC swine population.
References
Abyzov, A., Urban, A. E., Snyder, M., Gernstein, M. (2011). CNVnator: an arroyo to detect, genotype, and narrate typical and atypical CNVs from family unit and population genome sequencing. Genome Res. 21, 974–984. doi: 10.1101/gr.114876.110
PubMed Abstract | CrossRef Full Text | Google Scholar
Bailey, J. A., Kidd, J. M., Eichler, E. East. (2009). Man copy number polymorphic genes. Cytogenet. Genome. Res. 123 (one-4), 234–243. doi: 10.1159/000184713
CrossRef Total Text | Google Scholar
Baum, Grand. J., Cherry, J. A. (2015). Processing by the chief olfactory organization of chemosignals that facilitate mammalian reproduction. Hormones Behav. 68, 53–64. doi: 10.1016/j.yhbeh.2014.06.003
CrossRef Full Text | Google Scholar
Berglund, J., Nevalainen, East. M., Molin, A. M., Perloski, M., The LUPA Consortium, André, C., et al. (2012). Novel origins of copy number variation in the canis familiaris genome. Genome Biol. 13 (8), R73. doi: 10.1186/gb-2012-13-eight-r73
PubMed Abstract | CrossRef Full Text | Google Scholar
Bickhart, D. M., Hou, Y., Schroeder, S. G., Alkan, C., Cardone, Grand. F., Matukumalli, L. Chiliad., et al. (2012). Re-create number variation of individual cattle genomes using next-generation sequencing. Genome Res. 22 (4), 778–790. doi: ten.1101/gr.133967.111
PubMed Abstract | CrossRef Total Text | Google Scholar
Chen, C., Qiao, R., Wei, R., Guo, Y., Ai, H., Ma, J., et al. (2012). A comprehensive survey of re-create number variation in 18 diverse pig populations and identification of candidate copy number variable genes associated with complex traits. BMC Genomics 13 (1), 733. doi: 10.1186/1471-2164-xiii-733
PubMed Abstruse | CrossRef Total Text | Google Scholar
Choi, J.-W., Lee, Grand.-T., Liao, X., Stothard, P., An, H.-S., Ahn, Due south., et al. (2013). Genome-wide copy number variation in Hanwoo, Blackness Angus, and Holstein cattle. Mamm. Genome. 24 (three), 151–163. doi: 10.1007/s00335-013-9449-z
PubMed Abstract | CrossRef Full Text | Google Scholar
Conrad, D. F., Pinto, D., Redon, R., Feuk, L., Gokcumen, O., Zhang, Y., et al. (2010). Origins and functional affect of copy number variation in the human genome. Nature 464 (7289), 704–712. doi: 10.1038/nature08516
PubMed Abstract | CrossRef Full Text | Google Scholar
Crooijmans, R. P., Fife, Thousand. S., Fitzgerald, T. W., Strickland, S., Cheng, H. H., Kaiser, P., et al. (2013). Large calibration variation in Dna re-create number in chicken breeds. BMC Genomics xiv (1), 398. doi: x.1186/1471-2164-14-398
PubMed Abstruse | CrossRef Total Text | Google Scholar
Cross, A. J., Keel, B. Northward., Dark-brown-Brandl, T. M., Cassady, J. P., Rohrer, Chiliad. A. (2018). Genome-wide association of changes in swine feeding behavior due to heat stress. Genet. Sel. Evol. fifty, eleven. doi: ten.1186/s12711-018-0382-1
PubMed Abstract | CrossRef Full Text | Google Scholar
Duan, J., Deng, H.-W., Wang, Y.-P. (2014). Common copy number variation detection from multiple sequenced samples. IEEE Transact. Biomed. Engr. 61 (iii), 928–937. doi: 10.1109/TBME.2013.2292588
CrossRef Full Text | Google Scholar
Fadista, J., Nygaard, M., Holm, L.-E., Thomsen, B., Bendixen, C. (2008). A snapshot of CNVs in the hog genome. PLoS Ane 3 (12), e3916. doi: 10.1371/journal.pone.0003916
PubMed Abstract | CrossRef Full Text | Google Scholar
Feuk, Fifty., Carson, A. R., Scherer, Southward. W. (2006). Structural variation in the human genome. Nat. Genet. Rev. seven, 85–97. doi: x.1038/nrg1767
CrossRef Full Text | Google Scholar
Fontanesi, 50., Martelli, P. L., Beretti, F., Riggio, V., Dall'Olio, S., Colombo, M., et al. (2010). An initial comparative map of copy number variations in the goat (Capra hircus) genome. BMC Genomics 11 (1), 639. doi: x.1186/1471-2164-eleven-639
PubMed Abstract | CrossRef Full Text | Google Scholar
Fontanesi, L., Beretti, F., Martelli, P. L., Colombo, Thou., Dall'Olio, S., Occidente, M., et al. (2011). A kickoff comparative map of copy number variations in the sheep genome. Genomics 97 (three), 158–165. doi: 10.1016/j.ygeno.2010.xi.005
PubMed Abstruse | CrossRef Full Text | Google Scholar
Girirajan, S., Dennis, M. Y., Baker, C., Malig, Thou., Coe, B. P., Campbell, C. D., et al. (2013). Refinement and discovery of new hotspots of copy-number variation associated with autism spectrum disorder. Am. J. Hum. Genet. 92 (2), 221–237. doi: x.1016/j.ajhg.2012.12.016
PubMed Abstruse | CrossRef Full Text | Google Scholar
Groenen, M. A. M., Archibald, A. L., Uenishi, H., Tuggle, Due south. K., Takeuchi, Y., Rothschild, M. F., et al. (2012). Analyses of pig genomes provide insight into porcine demography and evolution. Nature 491 (7424), 393–398. doi: 10.1038/nature11622
PubMed Abstract | CrossRef Full Text | Google Scholar
Guryev, Five., Saar, K., Adamovic, T., Verheul, M., van Heesch, S. A. A., Cook, S., et al. (2008). Distribution and functional bear upon of DNA copy number variation in the rat. Nat. Genet. 40, 538–545. doi: x.1038/ng.141
PubMed Abstract | CrossRef Full Text | Google Scholar
Hay, E. H., Choi, I., Xu, Fifty., Zhou, Y., Rowland, R. R., Lunney, J. K., et al. (2017). CNV analysis of host responses to porcine reproductive and respiratory syndrome virus infection. J. Genomics 5, 58–63. doi: 10.7150/jgen.20358
PubMed Abstruse | CrossRef Full Text | Google Scholar
Henrichsen, C. N., Vinckenbosch, N., Zöllner, S., Chaignat, E., Pradervand, S., Schütz, F., et al. (2009). Segmental copy number variation shapes tissue transcriptomes. Nat. Genet. 41 (four), 424–429. doi: x.1038/ng.345
PubMed Abstract | CrossRef Total Text | Google Scholar
Hou, Y., Liu, M. E., Bickhart, D. M., Cardone, M. F., Wang, K., Kim, E. Southward., et al. (2011). Genomic characteristics of cattle copy number variations. BMC Genomics 12 (1), 127. doi: 10.1186/1471-2164-12-127
PubMed Abstruse | CrossRef Full Text | Google Scholar
Hou, Y., Bickhart, D. M., Hvinden, G. 50., Li, C., Song, J., Boichard, D. A., et al. (2012a). Fine mapping of re-create number variations on two cattle genome assemblies using high density SNP assortment. BMC Genomics xiii (1), 376. doi: 10.1186/1471-2164-thirteen-376
PubMed Abstract | CrossRef Full Text | Google Scholar
Hou, Y., Liu, Thou. Eastward., Bickhart, D. M., Matukumalli, Fifty. Yard., Li, C., Vocal, J., et al. (2012b). Genomic regions showing copy number variations acquaintance with resistance or susceptibility to gastrointestinal nematodes in Angus cattle. Funct. Integr. Genomics 12 (1), 81–92. doi: ten.1007/s10142-011-0252-1
PubMed Abstract | CrossRef Full Text | Google Scholar
Jiang, 50., Jiang, J., Wang, J., Ding, X., Liu, J., Zhang, Q. (2012). Genome-broad identification of re-create number variations in Chinese Holstein. PLoS Ane 7 (11), e48732. doi: 10.1371/journal.pone.0048732
PubMed Abstract | CrossRef Full Text | Google Scholar
Jiang, J., Wang, J., Wang, H., Zhang, Y., Kang, H., Feng, 10., et al. (2014). Global copy number analyses past side by side generation sequencing provide insight into pig genome variation. BMC Genomics xv, 593. doi: ten.1186/1471-2164-fifteen-593
PubMed Abstruse | CrossRef Total Text | Google Scholar
Keel, B. N., Keele, J. West., Snelling, W. M. (2016a). Genome-wide re-create number variation in the bovine genome detected using low coverage sequence of popular beef breeds. Anim. Genet. 48 (2), 141–150. doi: x.1111/age.12519
PubMed Abstract | CrossRef Total Text | Google Scholar
Keel, B. N., Lindholm-Perry, A. G., Snelling, Westward. M. (2016b). Evolutionary and functional features of copy number variation in the cattle genome. Front end Genet. vii, 207. doi: ten.3389/fgene.2016.00207
PubMed Abstruse | CrossRef Full Text | Google Scholar
Keel, B. N., Nonneman, D. J., Rohrer, G. A. (2017). A survey of single nucleotide polymorphisms identified from whole-genome sequencing and their functional effect in the porcine genome. Anim. Genet. 48 (4), 404–411. doi: 10.1111/historic period.12557
PubMed Abstruse | CrossRef Total Text | Google Scholar
Keel, B. N., Nonneman, D. J., Lindholm-Perry, A. K., Oliver, Westward. T., Rohrer, G. A. (2018). Porcine single nucleotide polymorphisms and their functional effect: an update. BMC Res. Notes. 11, 860. doi: 10.1186/s13104-018-3973-vi
PubMed Abstruse | CrossRef Full Text | Google Scholar
Klambauer, G., Schwarzbauer, K., Mayr, A., Clevert, D.-A., Mitterecker, A., Bodenhofer, U., et al. (2012). cn.MOPS: mixture of poissons for discovering copy number variations in side by side-generation sequencing data with a low false discovery rate. Nucleic Acids Res. twoscore (9), e69. doi: ten.1093/nar/gks003
PubMed Abstract | CrossRef Full Text | Google Scholar
Krzywinski, M., Schein, J., Birol, İ., Connors, J., Gascoyne, R., Horsman, D., et al. (2009). Circos: an information aesthetic for comparative genomics. Genome Res. 19 (9), 1639–1645. doi: 10.1101/gr.092759.109
PubMed Abstract | CrossRef Total Text | Google Scholar
Legault, Yard. A., Girard, S., Perreault, 50. P. 50., Rouleau, Yard. A., Dubé, M. P. (2015). Comparison of sequencing based CNV discovery methods using monozygotic twin quartets. PLoS One ten (iii), e0122287. doi: ten.1371/journal.pone.0122287
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, Y., Mei, S., Zhang, X., Peng, X., Liu, G., Tao, H., et al. (2012). Identification of genome-broad re-create number variations amid various pig breeds by array CGH. BMC Genomics 13 (1), 725. doi: 10.1186/1471-2164-13-725
PubMed Abstract | CrossRef Full Text | Google Scholar
Lindholm-Perry, A. K., Rohrer, Yard. A., Holl, J. W., Shackelford, Due south. D., Wheeler, T. J., Koohmaraie, K., et al. (2009). Relationships among calpastatin single nucleotide polymorphisms, calpastatin expression and tenderness in pork longissimus. Anim. Genet. twoscore, 713–721. doi: x.1111/j.1365-2052.2009.01903.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Lindholm-Perry, A. Yard., Rohrer, G. A., Kuehn., L. A., Keele, J. W., Holl, J. W., Shackelford, South. D., et al. (2010). Genomic regions associated with kyphosis in swine. BMC Genetics eleven, 112. doi: 10.1186/1471-2156-11-112
PubMed Abstract | CrossRef Full Text | Google Scholar
Liu, M. Due east., Hou, Y., Zhu, B., Cardone, M. F., Jiang, L., Cellamare, A., et al. (2010). Assay of copy number variations among diverse cattle breeds. Genome Res. 20 (5), 693–703. doi: 10.1101/gr.105403.110
PubMed Abstruse | CrossRef Full Text | Google Scholar
Liu, J., Zhang, 50., Xu, L., Ren, H., Lu, J., Zhang, 10., et al. (2013). Analysis of copy number variations in the sheep genome using 50K SNP BeadChip array. BMC Genomics 14 (1), 229. doi: x.1186/1471-2164-xiv-229
PubMed Abstract | CrossRef Full Text | Google Scholar
Locke, M. E. O., Milojevic, M., Eitutis, S. T., Patel, N., Wishart, A. E., Daley, M., et al. (2015). Genomic copy number variation in Mus muscle. BMC Genomics 16, 497. doi: x.1186/s12864-015-1713-z
PubMed Abstruse | CrossRef Full Text | Google Scholar
Long, Y., Su, Y., Ai, H., Zhang, Z., Yang, B., Ruan, G., et al. (2016). A genome-broad association study of copy number variations with umbilical hernia in swine. Anim. Genet. 47 (3), 298–305. doi: 10.1111/age.12402
PubMed Abstract | CrossRef Full Text | Google Scholar
Malekpour, S. A., Pezeshk, H., Sadeghi, M. (2018). MSeq-CNV: Authentic detection of copy number variation from sequencing of multiple samples. Nat. Sci. Rep. 8, 4009. doi: 10.1038/s41598-018-22323-eight
CrossRef Full Text | Google Scholar
Mi, H., Muruganujan, A., Casagrande, J. T., Thomas, P. D. (2013). Large-scale factor role assay with the PANTHER nomenclature organisation. Nat. Protoc. 8 (8), 1551–1566. doi: ten.1038/nprot.2013.092
PubMed Abstruse | CrossRef Total Text | Google Scholar
Nicholas, T. J., Baker, C., Eichler, Eastward. E., Akey, J. K. (2011). A high-resolution integrated map of copy number polymorphisms within and between breeds of the mod domesticated canis familiaris. BMC Genomics 12 (1), 414. doi: 10.1186/1471-2164-12-414
PubMed Abstract | CrossRef Total Text | Google Scholar
Nguyen, D. T., Lee, Chiliad., Choi, H., Choi, M. K., Le, M. T., Song, N., et al. (2012). The complete swine olfactory subgenome: expansion of the olfactory gene repertoire in the squealer genome. BMC Genomics thirteen, 584. doi: x.1186/1471-2164-13-584
PubMed Abstract | CrossRef Full Text | Google Scholar
Nonneman, D. J., Schneider, J. F., Lents, C. A., Wiedmann, R. T., Vallet, J. L., Rohrer, G. A. (2016). Genome-wide association and identification of candidate genes for age of puberty in swine. BMC Genetics 17, 50. doi: 10.1186/s12863-016-0352-y
PubMed Abstract | CrossRef Full Text | Google Scholar
Nonneman, D. J., Wiedmann, R. T., Snelling, Westward. M., Rohrer, One thousand. A. (2013). Genome-broad association of meat quality traits and tenderness in swine. J. Anim. Sci. 91, 4043–4050. doi: x.2527/jas.2013-6255
PubMed Abstract | CrossRef Total Text | Google Scholar
Paudel, Y., Madsen, O., Megens, H.-J., Frantz, 50. A., Bosse, One thousand., Bastiaansen, J. W., et al. (2013). Evolutionary dynamics of copy number variation in hog genomes in the context of accommodation and domestication. BMC Genomics fourteen (one), 449. doi: 10.1186/1471-2164-fourteen-449
PubMed Abstract | CrossRef Total Text | Google Scholar
Paudel, Y., Madsen, O., Megens, H.-J., Frantz, 50. A. F., Bosse, M., Crooijmans, R. P. Thou. A., et al. (2015). Copy number variation in the speciation of pigs: A possible prominent part for olfactory receptors. BMC Genomics 16 (one), 330. doi: 10.1186/s12864-015-1449-ix
PubMed Abstract | CrossRef Full Text | Google Scholar
Pezer, Ž., Harr, B., Teschke, M., Babikar, H., Tautz, D. (2015). Divergence patterns of genic copy number variation in natural populations of the house mouse (Mus musculus domesticus) reveal three conserved genes with major population-specific expansions. Genome Res. 25 (viii), 1114–1124. doi: 10.1101/gr.187187.114
PubMed Abstruse | CrossRef Full Text | Google Scholar
Ramayo-Caldas, Y., Castelló, A., Pena, R. Due north., Alves, E., Mercadé, A., Souza, C. A., et al. (2010). Copy number variation in the porcine genome inferred from a lx m SNP BeadChip. BMC Genomics 11 (1), 593. doi: 10.1186/1471-2164-11-593
PubMed Abstract | CrossRef Total Text | Google Scholar
Redon, R., Ishikawa, S., Fitch, Thou. R., Feuk, Fifty., Perry, Thousand. H., Andrews, T. D., et al. (2006). Global variation in copy number in the human genome. Nature 444, 444–454. doi: ten.1038/nature05329
PubMed Abstract | CrossRef Full Text | Google Scholar
Revay, T., Quach, A. T., Maignel, Fifty., Sullivan, B., Rex, W. A. (2015). Copy number variations in high and low fertility breeding boars. BMC Genomics xvi (i), 280. doi: 10.1186/s12864-015-1473-ix
PubMed Abstract | CrossRef Full Text | Google Scholar
Revilla, M., Puig-Oliveras, A., Castelló, A., Crespo-Piazuelo, D., Paludo, Eastward., Fernández, A. I., et al. (2017). A global analysis of CNVs in swine using whole genome sequence information and association analysis with fat acid composition and growth traits. PLoS Ane 12 (5), e0177014. doi: ten.1371/periodical.pone.0177014
PubMed Abstract | CrossRef Total Text | Google Scholar
Rohrer, K. A., Nonneman, D. J. (2017). Genetic analysis of teat number in pigs reveals some developmental pathways contained of vertebra number and several loci which but touch a specific side. Genet. Sel. Evol. 49, 4. doi: 10.1186/s12711-016-0282-1
PubMed Abstract | CrossRef Full Text | Google Scholar
Rohrer, G. A., Nonneman, D. J., Wiedmann, R. T., Schneider, J. F. (2015). A study of vertebra number in pigs confirms the association of vertnin and reveals additional QTL. BMC Genetics 16, 129. doi: x.1186/s12863-015-0286-9
PubMed Abstract | CrossRef Full Text | Google Scholar
Rohrer, Yard. A., Rempel, 50. A., Miles, J. R., Keele, J. W., Wiedmann, R. T., Vallet, J. L. (2014). Identifying genetic loci decision-making neonatal passive transfer of immunity using a hybrid genotyping strategy. Anim. Genet. 45, 340–349. doi: x.1111/age.12131
PubMed Abstract | CrossRef Total Text | Google Scholar
Schiavo, Yard., Dolezal, M. A., Scotti, East., Bertolini, F., Caló, D. G., Galimberti, Yard., et al. (2014). Re-create number variants in Italian Large White pigs detected using high-density single nucleotide polymorphisms and their association with back fat thickness. Anim. Genet. 45 (five), 745–749. doi: 10.1111/age.12180
PubMed Abstract | CrossRef Full Text | Google Scholar
Schneider, J. F., Miles, J. R., Brown-Brandl, T. K., Nienaber, J. A., Rohrer, G. A., Vallet, J. L. (2015). Genomewide clan assay for boilerplate birth interval and stillbirth in swine. J. Anim. Sci. 93, 529–540. doi: ten.2527/jas.2014-7899
PubMed Abstract | CrossRef Full Text | Google Scholar
Schneider, J. F., Nonneman, D. J., Wiedmann, R. T., Vallet, J. Fifty., Rohrer, G. A. (2014). Genomewide association and identification of candidate genes for ovulation rate in swine. J. Anim. Sci. 92, 3792–3803. doi: 10.2527/jas.2014-7788
PubMed Abstruse | CrossRef Full Text | Google Scholar
Schneider, J. F., Rempel, Fifty. A., Rohrer, G. A. (2012). Genome-wide clan study of swine farrowing traits. Part II: Bayesian analysis of marker data. J. Anim. Sci. xc, 3360–3367. doi: 10.2527/jas.2011-4759
PubMed Abstruse | CrossRef Full Text | Google Scholar
Spehr, Thousand., Schwane, K., Riffell, J. A., Zimmer, R. One thousand., Hatt, H. (2006). Odorant receptors and olfactory-like signaling mechanisms in mammalian sperm. Mol. Cell. Endocrinol. 250 (i–2), 128–136. doi: 10.1016/j.mce.2005.12.035
PubMed Abstract | CrossRef Full Text | Google Scholar
Stothard, P., Choi, J.-Westward., Basu, U., Sumner-Thomson, J. Grand., Meng, Y., Liao, Ten., et al. (2011). Whole genome resequencing of Black Angus and Holstein cattle for SNP and CNV discovery. BMC Genomics 12 (1), 559. doi: 10.1186/1471-2164-12-559
PubMed Abstract | CrossRef Full Text | Google Scholar
Stranger, B. E., Forrest, M. S., Dunning, M., Ingle, C. E., Beazley, C., Thorne, Northward., et al. (2007). Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science 315 (5813), 848–853. doi: 10.1126/science.1136678
PubMed Abstract | CrossRef Full Text | Google Scholar
Wang, H., Wang, C., Yang, K., Liu, J., Wang, Y., Xu, Ten., et al. (2015). Genome wide distributions and functional characterization of copy number variations betwixt Chinese and western pigs. PLoS One 10 (7), e0131522. doi: 10.1371/journal.pone.0131522
PubMed Abstract | CrossRef Full Text | Google Scholar
Wang, J., Jiang, J., Fu, W., Ding, X., Liu, J-F., Zhang, Q., et al. (2012). A genome-wide detection of copy number variations using SNP genotyping arrays in swine. BMC Genomics xiii (one), 273. doi: ten.1186/1471-2164-xiii-273
PubMed Abstract | CrossRef Full Text | Google Scholar
Wang, J., Wang, H., Jiang, J., Kang, H., Feng, X., Zhang, Q., et al. (2013). Identification of genome-broad copy number variations among diverse pig breeds using SNP genotyping arrays. PLoS One eight (7), e68683. doi: 10.1371/periodical.pone.0068683
PubMed Abstract | CrossRef Total Text | Google Scholar
Wang, J., Jiang, J., Wang, H., Kang, H., Zhang, Q., Liu, J. F. (2014). Enhancing genome-wide copy number variation identification past high density array CHH using various resource of pig breeds. PLoS One 9 (1), e87571. doi: 10.1371/journal.pone.0087571
PubMed Abstruse | CrossRef Full Text | Google Scholar
Wang, J., Jiang, J., Wang, H., Kang, H., Zhang, Q., Liu, J. F. (2015). Improved detection and characterization of copy number variations amid various sus scrofa breeds past assortment CGH. G3: Genes|Genomes|Genetics 5 (half dozen), 1253–1261. doi: 10.1534/g3.115.018473
CrossRef Total Text | Google Scholar
Wang, K., Li, M., Hadley, D., Liu, R., Glessner, J., Grant, S. F., et al. (2007). PennCNV: an integrated hidden Markov model designed for high-resolution copy number variation detection in whole-genome SNP genotyping data. Genome Res. 17, 1665–1674. doi: 10.1101/gr.6861907
PubMed Abstract | CrossRef Full Text | Google Scholar
Wang, L., Liu, X., Zhang, L., Yan, H., Luo, Due west., et al. (2013). Genome-wide copy number variations inferred from SNP genotyping arrays using a big white and Minzhu intercross population. PLoS One eight (10), e74879. doi: 10.1371/journal.pone.0074879
PubMed Abstract | CrossRef Full Text | Google Scholar
Wang, Z., Chen, Q., Liao, R., Zhang, L., Zhang, X., Liu, Ten., et al. (2016). Genome-broad genetic variation discovery in Chinese Taihu pig breeds using next generation sequencing. Anim. Genet. 48 (1), 38–47. doi: ten.1111/age.12465
PubMed Abstract | CrossRef Full Text | Google Scholar
Wiedmann, R. T., Nonneman, D. J., Rohrer, Thousand. A. (2015). Genome-wide copy number variations using SNP genotyping in a mixed breed swine population. PLoS 1 10 (vii), e0133529. doi: 10.1371/journal.pone.0133529
PubMed Abstract | CrossRef Full Text | Google Scholar
Wu, Y., Fan, H., Jing, S., Xia, J., Chen, Y., Zhang, L., et al. (2015). A genome-wide scan for copy number variations using high-density single nucleotide polymorphism assortment in Simmental cattle. Anim. Genet. 46 (3), 289–298. doi: 10.1111/age.12288
PubMed Abstruse | CrossRef Full Text | Google Scholar
Xie, J., Li, R., Li, South., Ran, Ten., Wang, J., Jiang, J., et al. (2016). Identification of re-create number variations in Xiang and Kele pigs. PLoS One 11 (ii), e0148565. doi: 10.1371/periodical.pone.0148565
PubMed Abstract | CrossRef Full Text | Google Scholar
Xu, L., Hou, H., Bickhart, D. M., Zhou, Y., Hay, E. H., Song, J., et al. (2016). Population-genetic properties of differentiated copy number variations in cattle. Sci. Rep. 6, 23161. doi: 10.1038/srep23161
PubMed Abstract | CrossRef Full Text | Google Scholar
Yi, G., Qu, Fifty., Liu, J., Yan, Y., Xu, G., Yang, North. (2014). Genome-wide patterns of copy number variation in the diversified chicken genomes using next-generation sequencing. BMC Genomics fifteen (1), 962. doi: 10.1186/1471-2164-15-962
PubMed Abstract | CrossRef Full Text | Google Scholar
Young, J. Chiliad., Endicott, R. K., Parghi, Southward. South., Walker, Grand., Kidd, J. Thousand., Trask, B. J. (2008). Extensive re-create-number variation of the human olfactory receptor gene family. Am. J. Hum. Genet. 83 (two), 228–242. doi: 10.1016/j.ajhg.2008.07.005
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhan, B., Fadista, J., Thomsen, B., Hedegaard, J., Panitz, F., Bendixen, C. (2011). Global assessment of genomic variation in cattle by genome resequencing and high-throughput genotyping. BMC Genomics 12 (1), 557. doi: 10.1186/1471-2164-12-557
PubMed Abstruse | CrossRef Full Text | Google Scholar
Zhao, Yard., Wang, Q., Wang, Q., Jia, P., Zhao, Z. (2013). Computational tools for copy number variation (CNV) detection using next-generation sequencing information: features and perspectives. BMC Bioinformatics 14, S1. doi: 10.1186/1471-2105-fourteen-S11-S1
CrossRef Total Text | Google Scholar
Zhou, 50. South., Li., J., Yang, J., Liu, C. 50., Xie, X. H., He, Y. North., et al. (2016). Genome-wide mapping of re-create number variations in commercial hybrid pigs using high-density SNP genotyping array. Genetika 52 (1), 97–105. doi: x.7868/S0016675815120140
PubMed Abstract | CrossRef Total Text | Google Scholar
Zheng, X., Shaffer, J. R., McHugh, C. P., Laurie, C. C., Feenstra, B., Melbye, M., et al. (2012). i.0 using family as a standard to evaluate copy number variation calling strategies for genetic association studies. Genet. Epidemiol 36 (3), 253–262. doi: 10.1002/gepi.21618
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhou, B., Ho, South. South., Zhang, 10., Pattni, R., Haraksingh, R. R., Urban, A. Due east. (2018). Whole-genome sequencing assay of CNV using depression-coverage and paired-end strategies is efficient and outperforms array-based CNV analysis. J. Med. Genet. xi, 735. doi: 10.1136/jmedgenet-2018-105272
CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fgene.2019.00737/full
0 Response to "Copy-number Variation (Cnv) Can Be Produced by: (Select All That Apply.)"
Post a Comment